
The Biomass Pyrolysis Spectrum
November 29, 2012
By Gerald Kutney
We use the pyrolysis of biomass every day without realizing it. The roasting and baking of foods are such processes.
We use the pyrolysis of biomass every day without realizing it. The roasting and baking of foods are such processes. Common pyrolysis reactors include toasters and barbecue grills. Caramel is produced by the pyrolysis of sugar, and another popular product is roasted coffee.
 |
|
| Figure 1. Biomass pyrolysis spectrum.
|
Pyrolysis is the thermal decomposition of biomass occurring in the absence of oxygen. The products of biomass pyrolysis (Figure 1 on page 22) include torrefied biomass, biocarbon, bio-oil and producer gas (methane, hydrogen, carbon monoxide and carbon dioxide). Depending on the thermal environment and the final temperature, pyrolysis will yield mainly biocarbon at low temperatures, less than 450˚C, when the heating rate is relatively slow, and mainly gases at high temperatures, greater than 600˚C, with rapid heating rates. At an intermediate temperature, but at higher heating rates, the main product is bio-oil.
The choice of one technology over another is often determined by the state-of-matter of the biofuel that is desired.
Torrefaction
The mildest pyrolysis process is torrefaction. During this reaction oxygen-rich compounds are volatilized from the biomass, including non-condensables such as carbon dioxide (80%) and carbon monoxide, and condensables such as water (60%), acetic acid (25%), methanol, formic acid and furaldehyde. These products mainly originate from hemicellulose, which is decomposed in the process. Torrefied wood is thus mainly composed of cellulose and lignin.
Torrefaction reaction rates are highest in straw, followed by hardwoods and softwoods, but the final properties are similar. The various reaction rates are attributed to the differing hemicellulose structures found in the different types of biomass.
The torrefaction reaction begins at 200˚C, but the practical range is 250˚C to 280˚C. In the torrefaction process, temperature is a more important factor than reaction time, which is typically 30 minutes or less. Care must be taken not to go much higher in temperature as carbonization begins in the range of 280˚C to 300˚C. The temperature is generally the major operational control parameter to determine the properties of the torrefied wood. Higher temperatures generally lead to higher energy densities, but yields decline. An optimum control point is autothermal operations where the energy of the volatiles is enough to supply the energy of the process.
When first invented, torrefied wood was often called “red charcoal” (charbon roux or Rothkohl) or “brown charcoal.” The word torrefaction, itself, is derived from the French word “torrefier,” which means “to roast.” Research on torrefaction began in France during the 1830s, and the self-binding property of torrefied wood was known by the turn of the 20th century; for example a U.S. patent was issued in 1901 to Joshua Gardner on the formation of briquettes of “partially-carbonized” sawdust. Another early American report of torrefaction was by Cleburne Basore in his Fuel Briquettes from Southern Pine Sawdust in 1929.
Today, interest has switched from briquettes to pellets, through development by firms such as ECN and Topell building the first torrefied wood pellet facility in Europe. Overall, when torrefied wood is pelletized, the physical properties are improved:
- The product is water-resistant: can be stored outdoors on a coal pile, and generally does not reabsorb moisture after drying.
- The fibrous nature is reduced and the grindability has been improved.
- Energy density is higher than that of a wood pellet.
- Bulk density is higher than that of a wood pellet, reducing transportation costs on an energy basis.
- No binder is necessary to form the pellet.
- Biological degradation is greatly reduced.
- Various biomass feedstock can be utilized.
- Uniform quality improves combustibility.
The targeted market for torrefied biomass is the coal-fired power sector. Torrefied wood is being used in Europe, and in North America, where trials have recently been carried out at the GWF Power Systems’ Pittsburg, Calif., petcoke power plant and at the James River power station in Springfield, Mo.
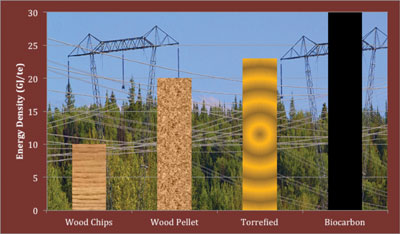 |
|
| Figure 2. The rise in higher-energy density.
|
As one goes from a wood chip to a wood pellet to a torrefied pellet to a biocarbon briquette, the general chemical and physical properties of the biomass are transformed more and more into a coal-like product. A generic technique to illustrate the chemical properties of various fuels compared to coal is to plot the hydrogen-to-carbon ratio versus the oxygen-to-carbon ratio of the fuel, which plot is called a Van Krevelen Diagram. The most obvious property is the rise in higher-energy density (Figure 2 on page 24).
Carbonization
Above 300˚C, carbonization of biomass commences and the thermochemical reactions become exothermic (i.e., heat-generating), which drives the higher-temperature pyrolysis with no (or little) external energy being applied. Biomass undergoes major chemical modifications at these higher temperatures.
Carbonization mimics coalification whereby nature converts plant matter into coal. Whereas coalification takes 300 million years, carbonization converts plant matter into charcoal, which has an energy density similar to bituminous coal, in 300 minutes (or less).
As the temperature of pyrolysis increases, mass is lost as oxygen-rich volatiles and the energy density of the remaining solid matter rises. Chemically, the percentage of carbon rises as the oxygen declines. The more carbon (and hydrogen) and the less oxygen that a material contains, the greater its energy density.
The higher energy density of charcoal compared to wood was one reason for its use in early industrial applications, such as blacksmithing, glassmaking and the iron industry. Currently, global production of charcoal is nearly 50 million tonnes.
A new market for charcoal is emerging as a renewable coal replacement for electricity generation. For industrial markets, the product is called biocarbon instead of charcoal; the latter term is reserved for recreational use. Biocarbon has another role to play in the fight against climate change as it can be used to sequester carbon in the soil. Biochar is the agricultural application of biocarbon. When applied to soil, biochar acts as an agricultural catalyst by promoting plant growth, but is not consumed. Since it is a catalyst, its benefits continue for generations to come without further addition. The biochar holds nutrients and fertilizers longer in the soil and provides other benefits encouraging plant growth. At the same time, the carbon has been sequestered or the carbon of the original biomass has been fixed and will not naturally return its carbon into the atmosphere. Biochar is one of the most promising agricultural breakthroughs since the discovery of fertilizers.
Producing biocarbon is achieved through the process known as slow pyrolysis. Heating the material very quickly, or fast pyrolysis, produces bio-oil, with biocarbon as a byproduct.
Gasification
Gasification is a pyrolysis process that creates producer gas, a low-energy gas containing mainly carbon monoxide and hydrogen (methane may also be present, especially at lower gasification temperatures). A pioneer in biomass gasification was Philippe Lebon (or Le Bon) D’Humbersin (1767-1804). In 1801, he put on a light demonstration in Paris that was fuelled by the gasification of wood. Unfortunately, the young entrepreneur was murdered three years later, before he could develop the technology further.
Biomass gasification technologies can be divided into three categories: fixed-bed, fluidized-bed and entrained-flow.
Fixed-bed gasifiers use a simple grate system whereby the process begins with a combustion zone that produces carbon dioxide and water; these products are then passed through a reducing zone to produce carbon monoxide and hydrogen. Fixed-bed reactors are usually utilized in systems of less than 5 MW. There are two types of fixed-bed gasifiers – updraft and downdraft – each of which has its advantages. Updraft gasifiers are a simple design but are prone to higher release of tars and other impurities. Downdraft gasifiers, while reducing the tar content, require a drier biomass feed and lose some of the biocarbon with the ash.
Fluidized-bed gasifiers are structurally more complex than fixed-bed, but generally produce a cleaner and more uniform producer gas. These facilities are usually in the range of five to 100 MW. Most larger-scale biomass gasification facilities have utilized fluidized-bed reactors, especially bubbling, which uses an inert heat-transfer medium such as sand.
Entrained-flow gasifiers are generally for larger units in the range of 50 to 500 MW and are the most efficient of the three technologies.
These processes can utilize two types of reactants, either air or steam.
Gasification has advantages over direct combustion or incineration of biomass, the most important of which is that the emissions are generally an order of magnitude lower than the emissions from an incinerator. As well, a more consistent fuel is sent to the boiler and ash is not carried directly into the boiler.
An important route to synthetic liquid fuels is related to the gasification process. The products of gasification, carbon monoxide and hydrogen, are called syngas when not contaminated by tars. Syngas can be converted into a variety of liquid fuels by the platform technology known as Fischer-Tropsch or FT technology. In 1925, Franz Fischer and Hans Tropsch discovered that syngas could be catalytically combined to form hydrocarbons (i.e., oil). Similar catalytic reactions can convert syngas into methanol or ethanol.
Conclusion
Although the products of the pyrolysis technologies vary greatly, they are all founded on a similar basic technology, the heating of biomass in a low-oxygen atmosphere. The separation between them depends on the temperature of the reaction. While the basic technologies have been known for over a century, only a few biomass-based facilities have been constructed, as the economics of these processes often cannot compete against those of fossil fuels. The result is that few plants have been constructed and fewer still have been a commercial success.
Gerald Kutney is managing director of Sixth Element Sustainable Management, an Ottawa-based consulting firm that provides executive services for investors and project developers in the area of bioenergy, biorefining, bioproducts and related biomass–based technologies.
Print this page